The largest group of carnivorous plants is in the plant order Nepenthales. From DNA studies we know the close relatives of the Nepenthales carnivores are the salt tolerant plant families Frankeniaceae (seaheath) and Tamaricaceae (tamarisk, salt cedar) plus Plumbaginaceae (plumbago, leadwort, sealavenders, seapinks) and Polygonaceae (buckwheat, knotweed, rhubarb, and sorrel). The salt tolerant species are noted for being able to live in salty soils near oceans and brackish water and excrete excess salt from their leaves.
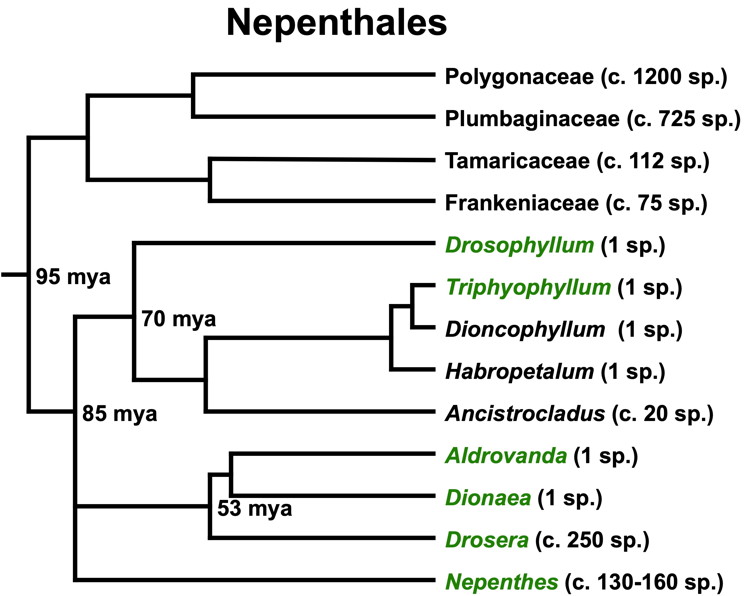
DNA cladogram of the plant order Nepenthales with the carnivores labeled in green and the non-carnivores in black. The length of the lines horizontally are proportional to the estimated time (mya = million years ago). The number of species in the family or genus are in parentheses. The figure is adapted from Fleischmann et al. (2018).
What does this cladogram tell us? We have mucilage-based tentacle trap plants closely related to snap traps, resinous tentacle traps related to pitfall trap plants, a genus of part-time carnivorous lianas, and a genus of non-carnivorous lianas all having a potentially carnivorous common ancestor. When did that common ancestor live, what did it look like, and how did we get from that ancestor to what we have now?
The pollen of Nepenthes, Drosera, Dionaea, and Aldrovanda is very distinctive. Using fossil pollen data plus fossils of more distantly related plants, Fleischmann et al. (2018) provide a timeline for the evolution of the Nepenthales carnivores. Their estimate for the root of the Nepenthales tree is about 95 million years ago. The estimate for the split of Drosophyllum, Drosera, and Nepenthes branches is about 85 million years ago. These dates are in the age of the dinosaurs. The estimate for split between Aldrovanda/Dionaea and Drosera is about 53 million years ago—12 million years after the extinction of the non-avian dinosaurs.
A World in Motion, Plants in Motion
Through continental drift, the land masses of this planet are constantly changing their climate. The plants have to shift and adapt or go extinct. That is part of the reason relic species tend to be found on mountains. It is easier to move up and down a mountain as climates shift than to migrate hundreds if not thousands of miles north and south. Sometimes the plants run out of mountain or continent. Antarctica had carnivorous plants on it 6 million years ago. Where are they now and were any of them key players evolutionarily?
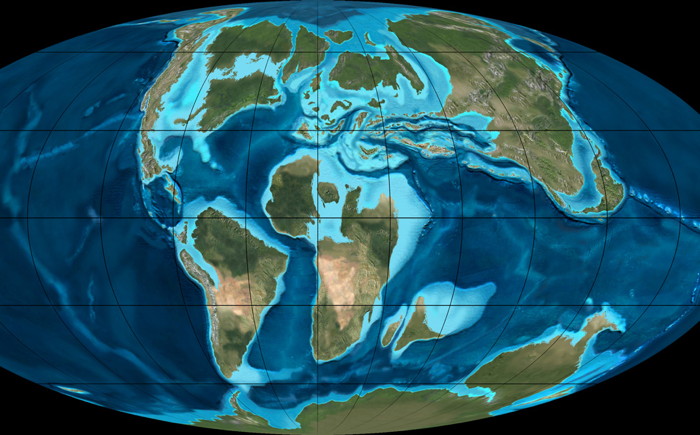
"Satellite view" of Earth in the late Cretaceous, 90 million years ago, during the age of dinosaurs and when the early Nepenthales species were evolving. Image © Ron Blakey, Northern Arizona University Geology.
The global changes that require plants to move around are partly a result in changes in the earth to sun distance, partly to changes in the angle of tilt of the planet relative to the sun, and partly a consequence of the configuration of continents. Only 15 thousand years ago, kilometer thick ice sheets extended well into North America, Europe, and Asia [Wikipedia] where there are whole communities of carnivorous plants today. Where were those plants during the ice age? (see Degreef 1989a PDF and Decoding Diversity: Glacial Stragglers for more on this topic)
To make matters even more interesting, there have been a number of mass extinctions [Wikipedia] caused by meteorite impacts and extreme volcanism. The Cretaceous–Tertiary global extinction event 66 million years ago [Wikipedia] killed more than the non-avian dinosaurs. The worst area hit was North America with over 50% of the plant species wiped out. Who knows how many species if not genera of carnivorous plants ceased to exist in that event. There were many less severe local extinction events caused by meteorites such as the one 14.8 million years ago [Wikipedia] that hit northern Europe. All these factors shape the life on this planet. They also make it hard for us to understand the details of how things evolved because so many pieces of the puzzle are missing.
There are two CPN review articles about Aldrovanda fossils that help determine evolutionary history of carnivorous Nepenthales. Degreef (1997 PDF ) reviewed publications on the fossil record of Aldrovanda seeds and pollen. (The fossils known as Paleoaldrovanda splendens have been shown to be insect eggs (Hermanova and Kvacek 2010). Ignore that part.) The oldest fossils of Aldrovanda itself were found in European Eocene age rocks, 38 to 55 million years old. At times since then there is evidence for at least 4 species extant at one time and at least 6 lineages going extinct. Schlauer (1997, PDF ) points out the record of a 6 million-year-old fossil leaf that appears to be Aldrovanda. We don't know what the older Aldrovanda species looked like. Since the seeds were discovered in aquatic sediments it is likely the plant was aquatic or semi-aquatic that whole time. (see Cross (2012) for a complete summary of the fossil data).
Fossil evidence indicates Nepenthes did not always live where they are found today. The oldest purported Nepenthes pollen discovered was in Eocene sediments deposited 40 to 56 million years ago just north of the Tethys sea in rocks now located in Europe (Krutzsh 1985). At that time proto-Europe was farther south and more tropical. These Nepenthes-like fossil pollen may actually be from a related but extinct species in the Nepenthales. Nepenthes-like fossil pollen has also been found on Kerguelen Island in the southern Indian Ocean indicating a southern origin. Either way, as the climate of Europe and southern Asia changed during the Miocene, 5 to 20 million years ago with the closing of the Tethys Sea, Nepenthes expanded their range into Southeast Asia and subsequently went extinct in Europe (Krutzsh 1989) or Africa or where they rafted north on the India continent. This was supported and expanded by the DNA results of Meimberg and Heubl (2006) (see Nepenthes phylogeny) where they found the current Madagascar, Seychelles, Sri Lanka, and India populations were founded by fully advanced Nepenthes before or simultaneously with the Southeast Asia populations 75 million years after the Nepenthes clade diverged from the Drosera and Drosophyllum clades. So, by the Miocene, about 8 million years ago, fully modern Nepenthes were migrating into Southeast Asia from south central Asia, plus there was a lot of migration between islands presumably as sea levels went up and down, resulting in approximately the 150 species we know today.
The earliest Drosera-like pollen fossils are known from Australia and Antarctica. Truswell and Marchant (1986) described pollen from central Australia they named Fischeripollis halensis. The fossil pollen was found in rocks dated to the early to mid Eocene near Alice Springs. Macphail and Truswell (2004) reported on pollen they called Fischeripollis sp. A. It was recovered in sediment cores drilled off the coast of Antarctica. When the images in these papers are compared to images in Takahashi and Sohma (1982) it appears Fischeripollis is Drosera and Fischeripollis sp. A is more ancestral-like than Fischeripollis halensis. These fossils show that Drosera as we know it was in Australia and Antarctica around 40 million years ago. Considering how little pollen Drosera species have in their flowers, that any is found is amazing! The vast majority of fossil pollen found in lake bottom cores is from wind pollinated flowers. In the case of Antarctic cores with Fischeripollis pollen, about a third of pollen in the cores was from Nothofagus-like (southern Beech tree) species.
Before discussing what the last common ancestor of the Nepenthales carnivores looked like, we need to look at the African carnivore Triphyophyllum peltatum. This drawing from a letter in a 1972 CPN ( PDF ) says it all:
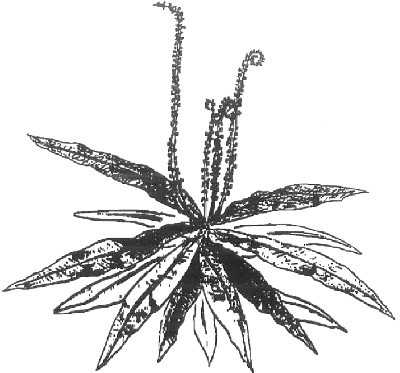
The young plant is like a pitcherless Nepenthes with some Drosophyllum leaves—those are not flowers pointing up. The carnivorous leaves with tentacles even unroll the way Drosophyllum does (reverse circinate) which is backwards from the way Drosera unrolls leaves. Drosera has it main digestive surface and most tentacles on the side of the leaf inside to the plant while Triphyophyllum and Drosophyllum have their primary digestive surface on the outer side of the leaf. The leaves unroll with the primary digestive surface inside to the coil.
The carnivorous leaves of Triphyophyllum do have a short, leaf-like petiole which makes the tentacular leaves analogous to the multi-part Nepenthes leaves. One difference is the pitchers of Nepenthes are constructed from leaves that fold in. Proto-Nepenthes must have had their primary digestive surface on the interior facing part of the leaves, unlike Triphyophyllum and Drosophyllum.
Mature Triphyophyllum are lianas [Wikipedia]. The leaves on the liana stems have grappling hooks on the tip where you would otherwise expect to find Nepenthes-like pitchers. The liana stems will occasionally supplement nutrition by putting out trapping leaves next to the grappling hook leaves. You can read more about this unbelievable plant in Bringmann et al. (1999 PDF , 2002 PDF ) and McPherson (2008).
There has been much discussion about whether the last common ancestor of the Nepenthales carnivores was pre-adapted for carnivory or a full-fledged carnivore. In other words, did carnivory evolve one, two, three or more times in the group? The question cannot be answered with any certainty. Since we are talking about a plant that lived more than 60 million years ago, we have to make a lot of inferences from what we see today. What we do know is the closest relatives of the Nepenthales carnivores today live in marginal habitats and many have glands for excreting salt or mucilage.
Can we see the roots of carnivory in the relatives of the Nepenthales carnivores? The Nepenthales carnivores did not just appear out of thick air. Nepenthes fanatics may think they recognize this image by Wilson (1890).
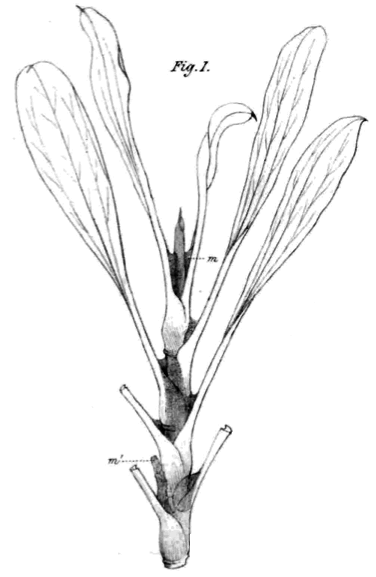
It looks exactly like a pitcherless Nepenthes such as Nepenthes rafflesiana. It isn't! The drawing is of the sea pink Limonium peregrinum found along the seashore in South Africa. This species is in the Plumbaginaceae and shows many leaf and gland characteristics common to the Plumbagos and to the Nepenthales carnivores. It has leaf bases that extend well around the stem. In some species these can get quite elaborate and pitcher like. This species has salt excretion glands in the base or petiole of the leaves and the dark material in the drawing is mucous secreted by those glands running down the stems. The leaf bases catch the exudates and may provide an effective barrier to crawling insects. This plant and its relatives display many leaf and gland characters that might be expected in the last common ancestor with the carnivores. They also display special adaptations that would have had many millions of years to evolve since they split from the carnivores. We cannot be certain what the plants were like 90 million years ago.
The glands of importance to compare across the Nepenthales carnivores are the sessile digestive glands (see figures to the right). The trapping tentacles of Triphyophyllum and Drosophyllum are a later development as are the multipurpose tentacles of Drosera. Drosera tentacles took over much of the original role of the sessile digestive glands. There is no common theme among the sessile gland across all the genera. This makes it more likely they became specialized for carnivory separately but is not proof.
The plants in the Drosera / Dionaea /Aldrovanda clade are all either aquatic or wetland while all the plants in the Nepenthes / Drosophyllum clade are adapted to well drained habitats. Some authors claim based on development and physiology that Aldrovanda, Dionaea, and Drosera appear to be derived from an aquatic or semi-aquatic ancestor (Degreef 1988 PDF ), there is no evidence of this for the Nepenthes or members of the Drosophyllum clade. It is easier to imagine the last common ancestor being more like the Nepenthes / Drosophyllum clade ancestors because they are more like typical plants with "normal" roots and leaves.
What about the last common ancestor between the Drosera and the Dionaea/Aldrovanda clades? Degreef (1989 , PDF ) has a good discussion of this. The most likely last common ancestor was a bog or semi-aquatic carnivorous plant. Based on the characteristics of the species extant today, how they misform leaves under stress conditions, and the kinds of glands and other structures on their leaves, that plant likely had the aspect of a sundew similar to Drosera arcturi (see Gibson 1999 PDF for more information on this species). The ancestor probably did not have tentacles but did have sessile digestive glands and maybe nectar glands on the leaves. The leaves more than likely were mobile and could close slowly on a prey. The specific characters we are looking at are the amplexicaul leaf bases of the more primitive species; most species, including Dionaea and Aldrovanda, regress to cuneate or spatulate leaves; all of them have similar sessile digestive glands on their leaves; and all have mobile leaves. All the genera have other glandular structures but the structure of the other glands is not the same between genera.
There are many hypotheses attempting to describe how Dionaea could have evolved from a relatively modern Drosera or other plant with tentacles. These stories, although clever, all require a set of unnecessary and extremely unlikely events and developmental changes. Degreef (1988 PDF ) lists a number of reasons with references to other scientific work. Essentially the analogous structures between Dionaea and Drosera result from having a common ancestor with common developmental pathways that produce derived structures that are superficially similar but very different in the fine details. The biggest objection is evolutionarily you can not get there from here. Carnivorous plants are carnivorous because that is the only way they can survive. To get from Drosera, which snags small insects on tentacles, to Dionaea, which traps larger flies between halves of a leaf, you have to go through intermediate forms with drastically reduced trapping efficiency. The scheme that makes most sense evolutionarily is both Drosera and Dionaea developed their trapping mechanisms in parallel under different environmental conditions.
Dionaea and Aldrovanda evolved their traps separately. If you look at how the snap traps of Dionaea and Aldrovanda operate, it is apparent that Aldrovanda is not an aquatic version of Dionaea. The traps of Aldrovanda work in a completely different way. Poppinga and Joyeux (2011) illustrated how the traps of Dionaea close by the outer layer of cells in the traps expanding to cause the trap walls to curve or cup closed. On the other hand the traps of Aldrovanda close at the hinge without changing the conformation of the trap walls. This implies the traps of the two plants evolved independently of each other. This was not known when Degreef wrote his CPN reviews.
How did the three part leaves of Nepenthes evolve? What we can look at to help us understand this is the leaves of seedling or juvenile plants. Some authors give the impression that Nepenthes pitchers evolved at the tips of tendrils. This is clearly not the case.
Mature Nepenthes leaves have three parts: the wide green petiole at the base, a tendril in the middle, and the pitcher at the end. In the youngest seedling leaves the petiole is very short and there is no tendril. The pitchers may be leafy pitchers or just like miniature adult pitchers. In slightly older seedlings, depending on the species, you can get a wide leafy petiole that grades into the pitcher or just a longer petiole. In yet older plants, a tendril forms between the petiole and the pitcher.
The current three part leaf structure appears to have developed as specializations for spreading out traps while the plants are in the rosette form and for grasping by the liana or vine form of the plant. The wide petiole increases the photosynthetic area and the tendril holds on to shrubs and other things while climbing. Owen and Lennon (1999) have a nice article showing how an adult pitcher develops and details of the glands.

Nepenthes rafflesiana juvenile leaves. Note on the left, the younger plant shows the petiole intergrades directly into the pitcher. A leaf from a more mature plant on the right shows the beginning of a clear separation of the petiole and the pitcher by a tendril.
Just like the other side of the family tree, understanding the evolution of adaptations used by the genera in the Nepenthes / Drosophyllum clade is difficult. It is essentially impossible for Triphyophyllum and Drosophyllum to have evolved from a plant we would recognize as a Nepenthes. Nepenthes plants are dioecious—each plant produces either male flowers or female female flowers—while Triphyophyllum and Drosophyllum plants have hermaphroditic flowers. Although it is possible a dioecious plant could evolve to have have both male and female flowers, to evolve flowers with both sexes would require the evolution of something unique. In addition, all Nepenthes species tested have been shown to be octoploid, having 8 sets of chromosomes, while Triphyophyllum is a tetraploid and Drosophyllum is a diploid.
It is also unlikely something like Triphyophyllum and Drosophyllum could give rise to Nepenthes. Both of the sticky-leaved plants are very specialized with their primary digestive leaf surfaces on different sides of the leaves from Nepenthes. The simplest explanation is a species with digestive glands on both the leaf surfaces split into two species that evolved in two different directions. One lineage folded its leaves in to protect the inner digestive surface from rain, the other formed tentacles on the outer surface.
A grain of salt for DNA Studies
DNA studies comparing species within genera help greatly in explaining the relationships among the existing species and give us hints as to paleogeography of the genus in general. However, even if all known species today are included in a DNA study there will be huge gaps in the results even beyond the technical issues of constructing trees. This is because it is extremely likely key species are extinct or otherwise missing from the data. As a result the cladograms can show spurious associations or apparent linkages that never actually occurred. All of the DNA testing results need to be verified with other physical data to make sure they make sense.
In reviewing the results of the DNA studies we have to keep in mind there has been a lot of hybridization between species in both Drosera and Nepenthes. The DNA sequence analysis software assumes independent evolution of the lineages studied. Hybridization produces data that violate this assumption. Most if not all species of Nepenthes can be crossed as well as many Drosera species can form hybrids. Hybridization results in plants that are a mix of the parents. If a study only looks at one or a few sequences or only plastid sequences, the results could indicate the hybrid is one or the other parent. If more sequences are added, especially if the sequences are nuclear, the resulting tree could show a lineage that never existed. If the study lumps nuclear and plastid sequences, the relationship trees can become quite distorted compared to trees with unlumped data.
We need to discount species counts because most Drosera species in Australia are self-incompatible and all Nepenthes species have only male or female plants. That is, for Nepenthes you need both a male and a female plant to get seeds. In the self-incompatible Drosera species, the flowers on any given plant can only be successfully pollinated from flowers of a plant that is sufficiently distantly related. That relative could easily be a different species. This results in many narrowly endemic species because an isolated plant is doomed not to reproduce by seeds. To make it more interesting, a Nepenthes or self-incompatible Drosera plant that migrates into the range of a different species could spawn a new species via hybridization with the resident species. The immigrant would not be able to reproduce itself but could inject new variation into the resident species allowing the local population to become different from the rest of the species.
The widespread Drosera species in Australia today and the Australian species that have migrated to New Zealand and to other continents are almost all self-compatible. All it takes is one seed to found a new population at a distant location. This is the sort of situation that is assumed by the DNA sequence analysis software.
Rivadavia et al. (2003) did a huge DNA study on Drosera species. In my mind it wasn't huge enough because it is missing some key species, but it did provide interesting results. I did an analysis in 2010 from publicly available data including all the data used by Rivadavia et al (2003). The results are on the Drosera Phylogeny page.
Based on the DNA data the most ancient species still in existence today are in South Africa (Drosera regia) and Australia/New Zealand (Drosera arcturi). Not all possible ancient species were tested so there may be more, especially in South America. This would confirm the pollen data and indicate an origin in southern hemisphere. During the cretaceous period the southern continents were closer together and without the Antarctic Circumpolar Current the climates were warmer so there could have been a lot of movement between the continents at that time. However it is not possible to rule out long-distance dispersal of Drosera regia much later. Either way, Drosera regia did not found the current Drosera flora of Africa. Drosera arcturi is found in sub-alpine regions of Australia, Tasmania, and New Zealand and does not have any close relatives either.
Australia has a very large number of Drosera species, many of them with narrow specializations. It is clear none of the woolly, tuberous, pygmy, or forked sundews contributed more than a species here or there to the flora of the other continents. The existence of a large number of species and/or forms in these groups and their mostly narrow endemic nature is most likely because of their self-incompatible flowers. Drosera glanduligera and Drosera burmannii are self compatible annuals, equally ancient, but didn't break up into dozens of species.
It is clear from Rivadavia's data that one or more relatively primitive Drosera migrated probably from Australia (and/or Antarctica) to the northern hemisphere via Asia. Separate migrants went to South America and to Africa relatively recently, possibly during the Miocene although it could be later. South America and North America subsequently exchanged a number of North America clade species back and forth. There is no clear species identified as the world colonizer in Rivadavia's study. However more recent data indicate that Drosera spatulata is a strong candidate as the species basal to most Asian, American, and African species. Those data also indicate that what we call Drosera spatulata may actually be a species group. More DNA studies need to be performed to better understand this species and the radiation out of Australia and/or Antarctica.
The African species (excluding Drosera regia) are so closely related Rivadavia could not make a definitive tree of their relationships. This could be because of a recent radiation into the continent or it could be a result of hybridization or both.
There were several intercontinental jumps noted producing sibling species. Drosera sessilifolia in eastern South America appears to be a very recent (for plants) disjunct from the Australian and south east Asian Drosera burmannii. Drosera uniflora in southern South America is a disjunct of the New Zealand Drosera stenopetala. There are a number of other species in South America that are either there by long distance dispersal or are relict species. One example is Drosera meristocaulis, which is found in northeastern South America. It is a basal or primitive pygmy Drosera with its closest relatives, including Drosera scorpioides, in Australia (Rivadavia et al. 2012).
-- John Brittnacher
January 2010
Latest update February 2025
For a more detailed discussion please see the following articles and articles they reference. Keep in mind each author states their view at a given time with the data they have at that time. Recent discoveries could modify their ideas.
Bringmann, Gerhard and Jan Schlauer and Kristina Wolf and Heiko Rischer and Uwe Buschbom and Andreas Kreiner and Friedrich Thiele and Martin Duschek and Laurent Ake Assi (1999) Cultivation of Triphyophyllum peltatum (Dioncophyllaceae), the part-time carnivorous plant. Carniv. Pl. Newslett. 28(1):7-13 ( PDF ) https://doi.org/10.55360/cpn281.gb418
Bringmann, Gerhard and Heiko Rischer and Jan Schlauer and Kristina Wolf and Andreas Kreiner and Martin Duschek and Laurent Ake Assi (2002) The Tropical Liana Triphyophyllum peltatum (Dioncophyllaceae): Formation of Carnivorous Organs is only a facultative prerequisite for shoot elongation. Carniv. Pl. Newslett. 31(2):44-52 ( PDF ) https://doi.org/10.55360/cpn312.gb193
Cameron, Kenneth, Kenneth J. Wurdack, and Richard W. Jobson (2002) Molecular evidence for the common origin of snap-traps among carnivorous plants. American Journal of Botany 89(9) 1503-1509. https://doi.org/10.3732/ajb.89.9.1503
Cross, A. (2012) Aldrovanda the Waterwheel Plant. Redfern Natural History Productions, Poole, Dorset, England.
Degreef, John D. (1988) The Evolution of Aldrovanda and Dionaea Traps. Carniv. Pl. Newslett. 17(4):119-125 ( PDF ) https://doi.org/10.55360/cpn174.jd944
Degreef, John D. (1989a) The Droseraceae during the glaciations. Carniv. Pl. Newslett. 18(2):45-46, 52-54 ( PDF ) https://doi.org/10.55360/cpn182.jd510
Degreef, John D. (1989b) Early history Drosera and Drosophyllum. Carniv. Pl. Newslett. 18(3):86-89 ( PDF ) https://doi.org/10.55360/cpn183.jd908
Degreef, John (1990a) Evolutionary patterns in Drosera. Carniv. Pl. Newslett. 19(1-2):11-16 ( PDF ) https://doi.org/10.55360/cpn191-2.jd316
DeGreef, John D. (1990b) More on the evolution of Drosera. Carniv. Pl. Newslett. 19(3-4):92 ( PDF ) https://doi.org/10.55360/cpn193-4.jd969
Degreef, John D. (1997) Fossil Aldrovanda. Carniv. Pl. Newslett. 26(3):93-97 ( PDF ) https://doi.org/10.55360/cpn263.jd244
Fleischmann, A., Schlauer, J., Smith, S. A., and Givnish, T. J. (2018) Evolution of carnivory in angiosperms. In: Carnivorous Plants: Physiology, ecology, and evolution. Edited by Aaron M. Ellison and Lubomír Adamec: Oxford University Press. https://doi.org/10.1093/oso/9780198779841.003.0003
Gibson, Robert (1999) Drosera arcturi in Tasmania and a comparison with Drosera regia. Carniv. Pl. Newslett. 28(3):76-80 ( PDF ) https://doi.org/10.55360/cpn283.rg548
Haberlandt, Gottlieb (from Sinnesorgane Im Pflanzenreich, Trans. By Carla R. Powell) (1982) Insectivores: Drosera and Drosophyllum. Carniv. Pl. Newslett. 11(3):66-73 ( PDF ) https://doi.org/10.55360/cpn113.gh920
Hermanova, Z. and J. Kvacek (2010) Late Cretaceous Palaeoaldrovanda, not seeds of a carnivorous plant, but eggs of an insect. Journal of the National Museum (Prague), Natural History Series, 179(9):105–118.
Heubl, G., G. Bringmann, and H. Meimberg (2006) Molecular phylogeny and character evolution of carnivorous plant families in Caryophyllales -- revisited. Plant Biology 8(2006):821-830. https://doi.org/10.1055/s-2006-924460
Jonathan (1992) A letter from Sierra Leone. Carniv. Pl. Newslett. 21(3):51-53 ( PDF ) https://doi.org/10.55360/cpn213.jj463
Krutzsh, W. (1985) Uber Nepenthes-Pollen (alias "Droseridites" p.p.) im europaischen Tertiar. Gleditschia 12:89-93.
Krutzsh, W. (1989) Paleogeography and historical phytogeography (paleochorology) in the Neophyticum. Pl. Syst. Evol. 162:5-61. https://doi.org/10.1007/978-3-7091-3972-1_2
Lledo, M. Dolores, Manuel B. Crespo, Kenneth M. Cameron, Michael F. Fay and Mark W. Chase (1998) Systematics of Plumbaginaceae Based upon Cladistic Analysis of rbcL Sequence Data. Systematic Botany 23(1):21-29. https://doi.org/10.2307/2419571
Macphail, M.K., and E.M.Truswell (2004) Palynology of Site 1166, Prydz Bay, East Antarctica. In Cooper, A.K., O’Brien, P.E., and Richter, C. (Eds.), Proc. ODP, Sci. Results, 188:1–43. ( PDF ) https://doi.org/10.2973/odp.proc.sr.188.013.2004
McPherson, Stewart (2008) Glistening Carnivores: the sticky-leaved insect-eating plants. Redfern Natural History Productions.
Meimberg H. and G. Heubl (2006) Introduction of a Nuclear Marker for Phylogenetic Analysis of Nepenthaceae. Plant Biology 8(2006):831–840. https://doi.org/10.1055/s-2006-924676
Müller, K. and Th. Borsch and L. Legendre and I Theisen and W. Barthlott (2002) Evolution of carnivory in the Lentibulariaceae: considerations based on molecular, morphological, and physiological evidence. Proc. 4th Intl. Carniv. Pl. Conf. pages 63-73 ( PDF )
Owen,T. P. Jr. and K. A. Lennon (1999) Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). American Journal of Botany 86(10):1382–1390 ( PDF ) https://doi.org/10.2307/2656921
Poppinga, Simon and Marc Joyeux (2011) Different mechanics of snap-trapping in the two closely related carnivorous plants Dionaea muscipula and Aldrovanda vesiculosa. Physical Review E 84, 041928. DOI: 10.1103/PhysRevE.84.041928 https://doi.org/10.1103/PhysRevE.84.041928
Rembold, Katja, Andreas Irmer, Simon Poppinga, Heiko Rischer and Gerhard Bringmann (2010) Propagation of Triphyophyllum peltatum(Dioncophyllaceae) and observations on its carnivory. Carniv. Pl. Newslett. 39(3):71-77. https://doi.org/10.55360/cpn393.kr694
Rivadavia, Fernando and Katsuhiko Kondo and Mitsuyasu Hasebe (2002) Phylogeny of the sundews, Drosera (Droseraceae) based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. Proc. 4th Intl. Carniv. Pl. Conf. pages 9-13 ( PDF ) (incomplete)
Rivadavia, Fernando, Katsuhiko Kondo, Masahiro Kato, and Mitsuyasu Hasebe (2003) Phylogeny of the sundews, Drosera (Droseraceae) based on chloroplast rbcL and nuclear 18S ribosomal DNA sequences. American Journal of Botany 90(1):123-130. https://doi.org/10.3732/ajb.90.1.123
Rivadavia, F., V. F. O. de Miranda, G. Hoogenstrijd, F. Pinheiro, G. Heubl and A. Fleischmann (2012) Is Drosera meristocaulis a pygmy sundew? Evidence of a long-distance dispersal between Western Australia and northern South America. Ann Bot 110(1): 11-21. https://doi.org/10.1093/aob/mcs096
Schlauer, Jan (1997) 'New' data relating to the evolution and phylogeny of some carnivorous plant families. Carniv. Pl. Newslett. 26(2):34-38 ( PDF ) https://doi.org/10.55360/cpn262.js506
Schlauer, Jan (1997) Fossil Aldrovanda -- Additions. Carniv. Pl. Newslett. 26(3):98 ( PDF ) https://doi.org/10.55360/cpn263.js888
Stoltzfus, A. and J. Suda and R. Kettering and A. Wolfe and S. Williams (2002) Secretion of digestive enzymes in Plumbago. Proc. 4th Intl. Carniv. Pl. Conf. pages 203-207 ( PDF )
Takahashi, H. and K. Sohma (1982) Pollen morphology of the Droseraceae and its related taxa. Sci. Rep. Tohoku Univ., IV 38(2):81-156.
Truswell, E.M., and N.G. Marchant (1986) Early Tertiary pollen of probable Droseracean affinity from central Australia. Spec. Pap.—Palaeontol., 35:163–178.
Wilson, John (1890) The mucilage- and other glands of the Plumbagineae. Ann. Bot. 4:231-258. https://doi.org/10.1093/oxfordjournals.aob.a090560
Yesson, C. and A. Culham (2006) Phyloclimatic Modeling: Combining Phylogenetics and Bioclimatic Modeling. Systematic Biology 55(5):785-802. https://doi.org/10.1080/1063515060081570

The murderous plant Plumbago auriculata in the family Plumbaginaceae, has stalked glands that will trap and kill small organisms—usually crawling insects attempting to get to the flowers. It can be induced to secrete digestive enzymes (Stoltzfus et al. 2002 PDF) but not in a way that would be typical of a true carnivore.
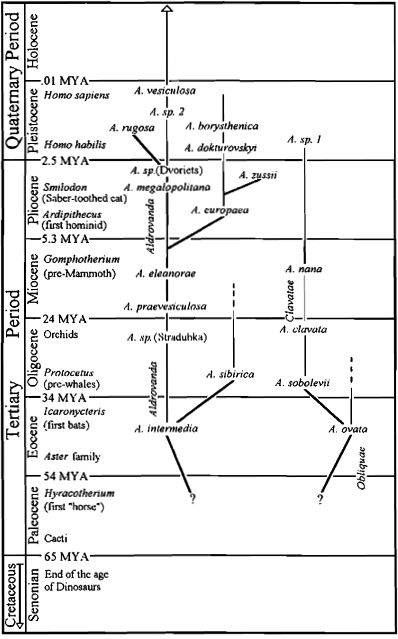
Evolution of Aldrovanda. Original figure by Barry Rice and Jan Schlauer based on references published in article by Degreef (1997 PDF ). Fossils attributed to Paleoaldrovanda splendens are now believed to be fossil insect eggs (Hermanova and Kvacek 2010). This "species" was removed from this version of the figure.

Aldrovanda vesiculosa.
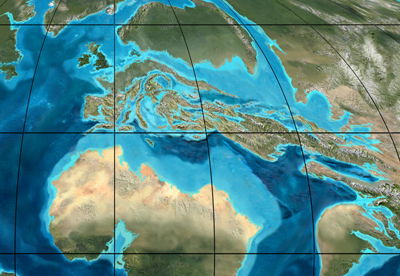
"Satellite view" of Europe in the Eocene, 50 million years ago. This is about the time the first Aldrovanda seeds were turned to stone on islands that later became Europe as we know it. It is also the approximate date of the Aldrovanda/Dionaea and Drosera split. Image © Ron Blakey, Northern Arizona University Geology.
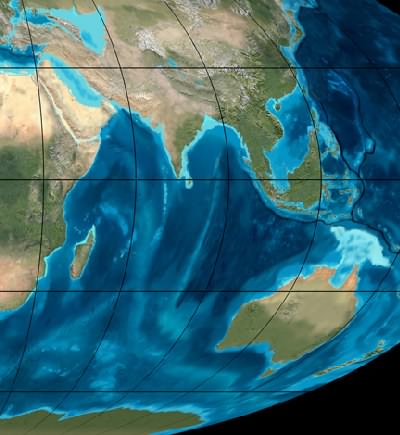
Miocene "Satellite view" of the Indian Ocean about 20 million years ago. This is the time many plants were "migrating" as a result of climate change on migrating continents. Note Antarctica is ice free along the coast. Image © Ron Blakey, Northern Arizona University Geology.

Nepenthes ventricosa.

Triphyophyllum peltatum with three glandular leaves in the greenhouse of the Botanical Gardens of Bonn. Cover photo from September 2010 CPN issue (Rembold et al. 2012). Photo by Katja Rembold.
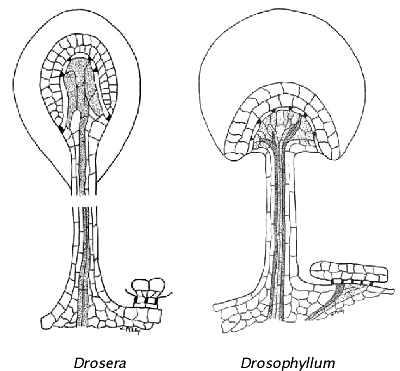
Figure from Haberlandt (1982 PDF ) drawn by Wayne Perry. Notice the different structure of the tentacles and sessile glands. The Drosophyllum sessile gland is vascularized for quick and copious release of mucilage while the Drosera sessile gland is not.
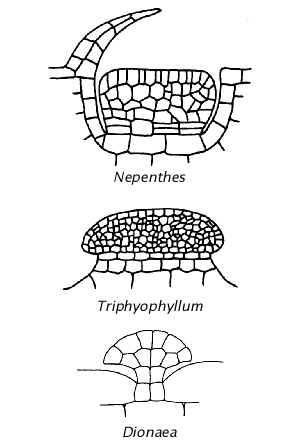
Drawings not to scale of digestive glands from Müller (2002 PDF ) reproduced from other sources. Note the The Nepenthes and Triphyophyllum digestive glands are similar to the Drosophyllum digestive gland although they are not vascularized. In an actual pitcher the Nepenthes gland is vertical and is recessed with a flap of cells over the gland that prevents a prey from getting a foothold on the gland. A Triphyophyllum-like gland would give a prey an escape ladder. The Dionaea digestive gland looks like it could evolve into a tentacle but is not vascularized like the Drosera tentacle.
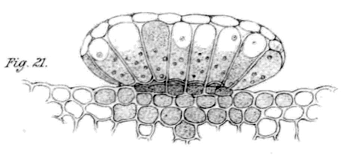
Salt excretion gland of Limonium peregrinum (Statice rosea in paper) as drawn by Wilson (1890).
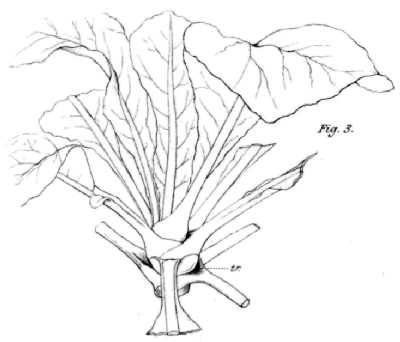
Limonium fruticans from the Canary Islands also drawn by John Wilson (1890) showing troughs at the amplexicaul leaf bases accumulating mucous.

Nepenthes aristolochioides pitcher.

Dionaea muscipula.

Dionaea muscipula traps.

Drosera regia leaf. Note the large tentacles and small red sessile glands on the leaf surface.

Drosera regia from South Africa (growing in captivity). The plants are about 40 cm tall. Drosera regia is so different from other Drosera, it was proposed in 1996 that it be renamed Freatulina regia. This name change has not been accepted by the CP community.

Rosette phase of the Australian Drosera peltata.

Juvenile leaves of Drosera binata from Tasmania. Eventually the plants will have the typical forked leaves for which the species is named.

Drosera spatulata from southern New Zealand. The plant is about 20 mm wide.

Drosera spatulata from Hong Kong. The plant is about 35 mm wide.

Drosera tokaiensis from Japan. This species is of hybrid origin. The parents appear to be Drosera spatulata and Drosera rotundifolia.

Drosera hirtella from South America.

Drosera dielsiana from South Africa.

Drosera brevifolia from North America. This warm temperate species is closely related to the cold temperate (hibernacula-forming) D. rotundifolia.

Drosera rotundifolia found across the northern hemisphere.